THE SOMATOSENSORY SYSTEM: OVERVIEW
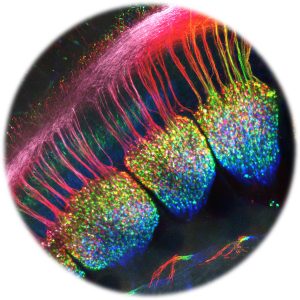
The somatosensory system processes information that organisms “feel”. Somatosensation refers to the bodily senses of limb position (proprioception), touch (mechanosensation), itch (pruriception), temperature (thermosensation) and noxious thermal, mechanical and chemical stimuli (nociception). The system is composed of a large number of distinct primary sensory neurons that are specialized to detect specific stimuli. Primary somatosensory neurons have their cell bodies in chains of ganglia adjacent to the spinal cord (the dorsal root ganglia) and at the base of the skull, anterior to the ear (the trigeminal ganglia) in the head. They are pseudounipolar neurons, with one unique process that bifurcates into two branches. One proximal branch projects to cutaneous or deep peripheral tissues. The other one enters the dorsal horn of the spinal cord or the brain stem where they synapse to other neurons that relay sensory signals to the brain, or directly lead to motor responses.
In the trunk, somatosensory neurons develop from a common pool of embryonic pluripotent cells, the neural crest. While significant progress have been done in the last decades in our understanding of the genetic control of somatosensory development, how this neuronal diversity and functional specialization is established during embryogenesis and what controls their functional properties at adult stage remains incompletely understood. Our laboratory has currently projects on nociceptors and mechanoreceptors.
- NOCICEPTORS
By initiating pain, the somatosensory system has an important alarm function, and is therefore essential for our survival. Pain is also causing untold misery and suffering when the pain-sensing system becomes over- or erroneously activated. Pain results from the activation of a subset of somatosensory neurons called nociceptors that are specialized to detect potentially harmful stimuli such as scalding heat, injurious mechanical forces and irritants. An important property of nociceptors is that they sensitize (that is, their excitability can be increased). Sensitization is defined as a reduced threshold to the stimulation required to generate a response. This can lead to allodynia (a stimulus outside of the nociceptive range generating a nociceptive response) or hyperalgesia (a nociceptive stimulus generating an exacerbated nociceptive response). Sensitization of nociceptors develops as a consequence of either inflammation at the site of tissue injury or nerve injury. It is one of the key processes leading to chronic pain, a major public health burden, estimated to affect one fifth of the population worldwide. Few patients with chronic pain obtain complete relief from drugs that are currently available, and more than half reports inadequate relief. There is thus an important need for more effective analgesics. The development of such innovative pain therapeutics requires a better understanding of the complex and heterogeneous mechanisms leading to the development of pain as a pathological condition.
Congenital insensitivity to pain (CIP) is a rare genetic disorder rendering individuals unable to feel pain since birth. Only a small number of CIP causing genes are known, and their discovery has led to profound insights into nociception. Among them, Prdm12, encoding an evolutionarily conserved zinc finger epigenetic regulator of gene expression. Our previous work on Prdm12 has shown that during neurogenesis, Prdm12 acts a master regulator of the entire nociceptive lineage. More recently, we found that in mature nociceptors in adult mice, Prdm12 regulates nociceptor excitability. We are currently studying the mechanisms by which Prdm12 promotes in neural crest sensory precursors a nociceptive fate and how it contributes to the control of nociceptor excitability at adulthood. Results of our work may provide useful clues for the development of novel pain therapeutic strategies.
- MECHANORECEPTORS
The primary sensory neurons that detect and propagate information about our surrounding tactile environment, particularly non-painful and pleasurable touch, are termed low threshold mechanoreceptors (LTMR) as they react to innocuous mechanical stimulations. A number of subtypes of LTMRs have been identified that give rise to the broad spectrum of touch sensation we experience but much remains to be understood about their development and diversification. We are currently investigating the role of several candidate players in the specification and wiring of tactile sensory neurons.

